Introducing DGXPComplianceLab®, Your Reliable Partner in Life Sciences Consulting.
At DGXPComplianceLab®, we are more than just consultants; we are your strategic allies in navigating the dynamic landscape of the Pharmaceutical/Clinical trials/Medical devices industry. With a relentless commitment to excellence and innovation, we offer a comprehensive suite of consulting services tailored to meet the diverse needs of companies worldwide.
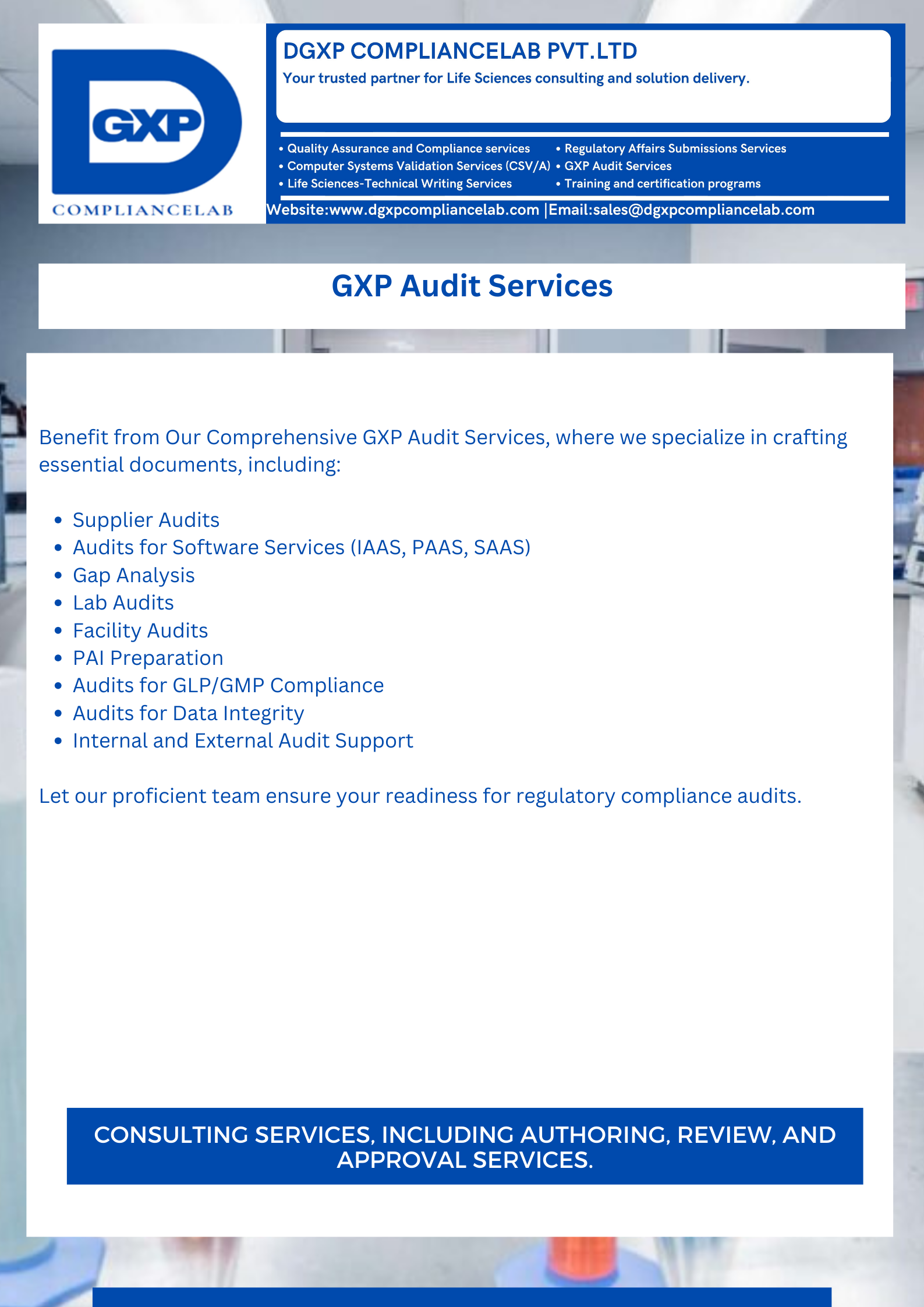
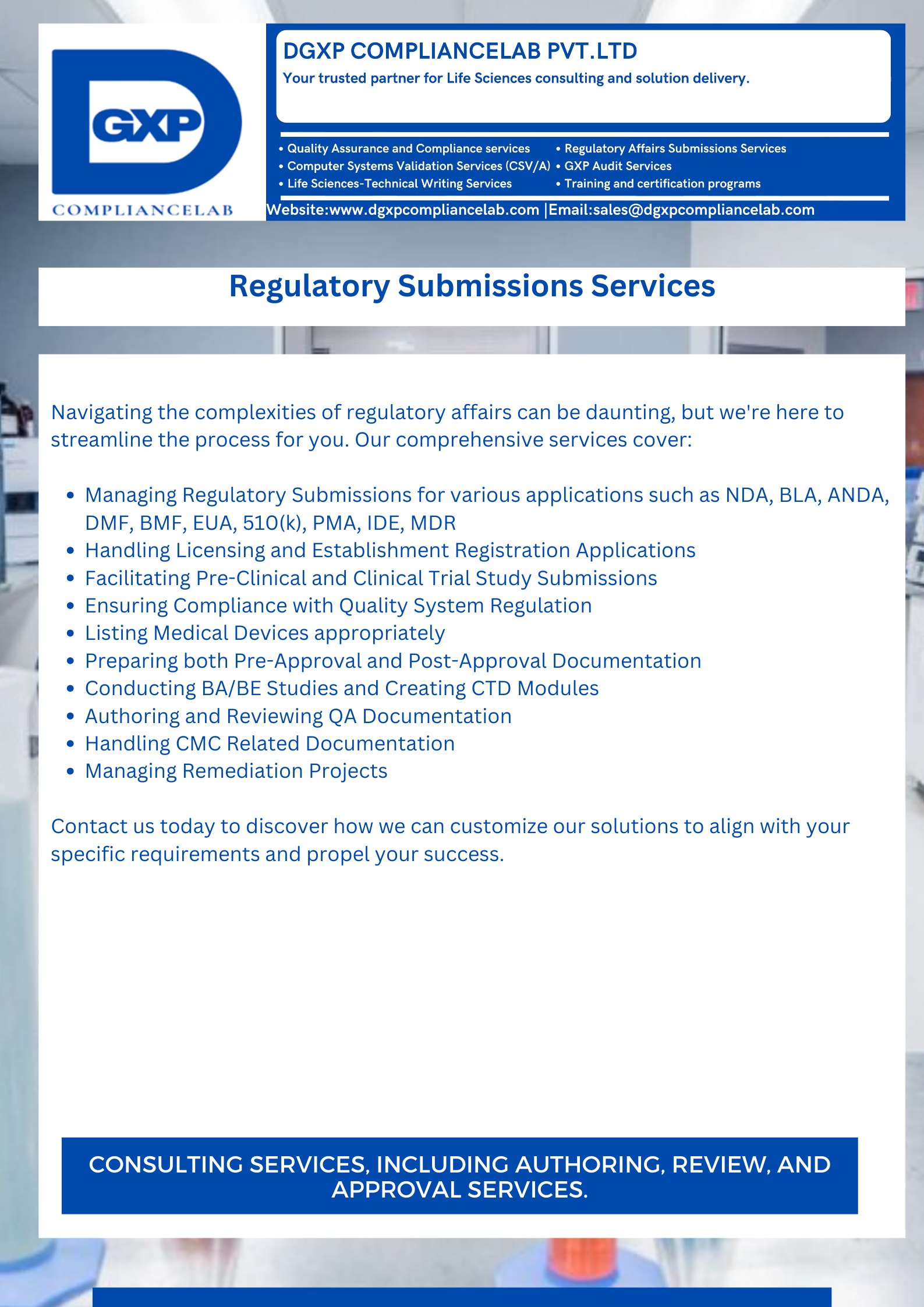
Welcome to our Life Science Solutions! We specialize in Computer system validation, Technical writing, Quality Assurance, Comprehensive GXP audit services, Digital transformation, and Management of regulatory submissions across various industries, including:
- Pharmaceutical
- Clinical Trials
- Medical Devices
- Nutraceuticals
At DGXPComplianceLab®, we offer scalable Life Science Consulting services tailored to meet our clients' specific needs. Whether you require the expertise of a support technician or an experienced Subject Matter Expert, or if you need a single consultant or a Managed Services Team, we are dedicated to delivering each project with unparalleled professionalism and quality that you can rely on.
Our commitment is to provide cost-effective solutions while delivering exceptional technical capabilities and customer service to all our clients, regardless of project size or complexity.
Allow us to guide you through the intricacies of the life sciences industry with our expertise and tailored solutions.
We specialize in Computer system validation, Technical writing, Quality Assurance, Comprehensive GXP audit services, Digital transformation, and Management of regulatory submissions across various industries including: Pharmaceutical, Clinical Trials, Medical Devices, Nutraceuticals.